During the manufacturing process, our team of engineers often discuss questions: What is the melting point of silicone? What temperatures can it withstand? What is the temperature at which degradation begins?
If you must get a precise answer, please inform the actual use environment of the silicone and its final contact temperature.
Silicone is known for its stable structure, thermal stability, and flexibility. Unlike most plastics that slowly melt at temperatures above 80°C, it can be exposed to a wide operating temperature range of -60°C (-76°F) to +300°C (572°F) and Will not deform. This makes it a durable material from consumer products to demanding aerospace applications.
In this article, we provide a basic guide to the melting point of silicone and answer related questions including the structure of silicone, melting point, burning point and the temperature range of different grades of silicone. You will learn more useful information on this topic as well as the key factors that influence the melting point of this compound, how to regulate the melting point.
Let’s first review the definition of silicone and its manufacturing process.
What is silicone?
Silicone, also known as silicone rubber, is an inert, rubber-like silicone elastomer made from siloxane polymers containing oxygen, silicon, hydrogen and carbon. It retains most of the beneficial properties of silicone, such as temperature resistance and chemical stability. It is usually obtained by adding various raw materials to obtain additional properties, such as mechanical strength, longer service life and higher heat resistance.
Chemical structure
It is an elastomer made from silicone-containing polymers. The silicon atoms in these rubber-like silicone polymers contain carbon-based substituents, with the primary family member being methyl. In addition, the remaining valence states of silicon can be linked to phenyl, vinyl, or hydrogen to provide additional specific properties.
What is silicone made of? How is it made?
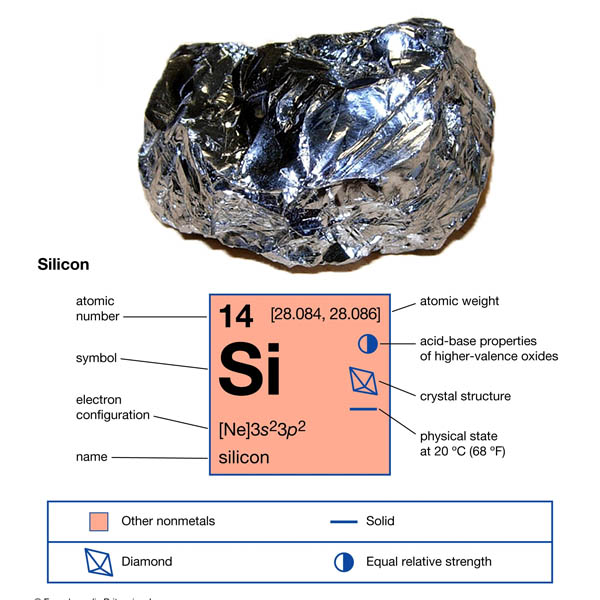
When discussing the melting point of silicone, first understand the manufacturing process of this polymer and the basic steps.
Silicon is a naturally occurring chemical element, but it rarely occurs in nature in pure elemental form. Metallic silicon is separated from ordinary sand or rocks . This is achieved by heating large amounts of quartz sand together with a copper catalyst to a high temperature of 1800C°. The silicon is pulverized and evenly mixed with methyl chloride to obtain various organosilicon intermediates, which It is then converted into polysiloxane compounds through different synthesis stages. These silicone polymers are mixed with additives and reinforcing fillers into a homogeneous, uncured material known as the silicone base stock. The final composition is tailored to the properties required for the product application. Finally, the silicone base compound is shaped and vulcanized into the final shape through different molding processes.
Will silicone melt?
Although silicone does not melt in extreme temperature applications, it does melt when it reaches certain temperature conditions. Below we further elaborate on the chemical element silicon from which silicone rubber is made.
It is generally known that silicon, the second most abundant element in the earth's crust, has an extremely high melting point, as high as 1414°C (2577.2°F), which means that silicone will melt when given the right temperature. Covalent bonds between atoms in molecules provide strong interaction forces. These covalent bonds break when melted and lose most or all of their strength. In other words, if you want to melt silicone, you need to provide enough heat (energy) to break these bonds. This is the fundamental reason why silicone will not melt in regular high-temperature applications.
Will silicone burn?
The natural point of silicone is around 450C°. Silicone that is generally in contact with our lives will not burn at high temperatures, but when the temperature reaches or exceeds when it ignites, the silicone will begin to emit toxic smoke, expand in volume, and finally produce a white flame. This process releases some toxic gases such as carbon monoxide. In addition, the organic groups in its molecules will be lost under the action of relatively high temperatures. Self-extinguishing silica (white powder) will remain in the burning area.
Silicone melting point
Unlike most polymers that begin to melt at high temperatures, silicone's temperature range is considered to be 200°C to 300°C. It exhibits unique behavior in extreme (high temperature) environments. It has no or almost no melting point and remains solid until burned. In addition, at 200 to 450 degrees Celsius, silicone gradually loses some of its properties over time, such as mechanical properties. At the same time, it become less elastic. At this point you will be surprised to find that silicone does not melt due to temperature.
What is the effect of heating on silicone?
Most industrial materials begin to melt when exposed to high temperatures. Silicone can withstand a wide range of temperatures in industrial applications and often does not reach its burn and melting points. However, heating does have an impact on the application performance and service life of silicone.
Simple example:
For continuous temperatures up to 150C°, silicone shows little change.
When exposed to temperatures of 200C° and over time, silicone begins to lose some of its mechanical properties and elasticity.
In high temperature applications exceeding 300C°, the material may degrade within a very short service life. It will become stiffer and less elastic. But it still remains solid.
Temperature range of different grades of silicone
General purpose type - also known as general purpose silicone, generally has temperature restrictions. Generally its operating temperature range is from as low as -50C°(-58°F) to as high as 250 °C(482 °F).
Heat stable type - also known as heat stable silicone, is made by adding special additions. Its maximum temperature increases to approximately 260C° (500 °F).
High temperature type - also known as high temperature silicone, is made by adding heat-resistant additives. It can be exposed non-continuously to temperatures up to 300C°(572 °F). and performs well. But this will reduce its performance and shorten its service life.
Flame retardant type - also known as flame retardant silicone, contains self-extinguishing additives. It is suitable for intermittent use at temperatures up to 220C°(428 °F).. It is also available in different grade options based on your project application to meet some international standards such as UUL94-VO, EN45545-2.
Silicone has such strong temperature resistance because it has high bond energy covalent bonds.
Key factors affecting the melting point of silicone
The melting point of silicone ranges from 537.778°C to 1414°C. This depends on its structure and the forces between molecules. Factors that affect melting temperature typically include the following:
Usage grade: The introduction of organic groups will destroy the stability of the silicon-oxygen bond and lower the melting point of the silica gel. Therefore, choosing different grades of silicone is crucial for the high-temperature application of your project. For example: Phenyl silicone rubber has a higher melting point compared to methyl silicone rubber.
Purity: The melting point of silicone is affected by impurities. The presence of impurities will interfere with the interaction between molecules and lower the melting point.
Additive type: The addition of plasticizer can weaken the interaction between polymer chains and lower the melting point. Adding an appropriate amount of filler can increase the melting point of the material. But excess will lower the melting point.
Molecular chain length: Generally speaking, the longer the molecular chain of silicone, the stronger the interaction between molecules, and the melting point will increase accordingly.
How to control the melting point of silicone?
There are three common ways to adjust the melting point of silicone:
First: Use different grades of silicone
Second: Adding fillers which change the composition of silicone
Third: Use different curing processes
Summarize
As you can see from the above, silicone will undergo some changes (loss of mechanical properties, decomposition) before it reaches its melting point. Its specific melting point will vary depending on the grade (type) of silicone used in your project. Now that you have the most comprehensive knowledge about the melting point of silicone, you can choose the most suitable silicone for your project application. If you still have questions about the melting point of silicone or need silicone project support, please contact our professional team.

